* * *
* * *
* * *
im·mu·no·glob·u·lin (im″u-no-globґu-lin) any of the structurally related glycoproteins that function as antibodies, divided into five classes (IgM, IgG, IgA, IgD, and IgE) on the basis of structure and biologic activity. The basic structural unit of the immunoglobulin molecule, referred to as a monomer, is a Y-shaped molecule composed of two heavy (H) chains and two light (L) chains (see illustration). IgD, IgG, and IgE occur only as monomers; IgM and IgA may occur as monomers or polymers. The polymeric forms contain an additional polypeptide called the J chain, and secretory IgA contains another structure called the secretory component (SC). Each chain consists of a variable region (VH or VL) and a constant region (CH or CL), which are coded for by different genes. Parts of the VH and VL regions make up the antigen-binding site, one on each “arm†(Fab region) of the monomer. An individual can make about 104 different VH regions and 103 VL regions, which combine to make about 107 different antigen-binding sites, each with a distinct antigenic specificity. Parts of the CH regions make up the “body†(Fc region) of the monomer, which contains various sites responsible for the biological activity of the molecule. In any one immunoglobulin molecule, all of the H chains are identical, as are the L chains. The CH region determines both the heavy chain class to which the H chain belongs and the immunoglobulin class to which the molecule belongs. The H chain classes are denoted by the Greek letters (μ, δ, γ, ε, and α) corresponding to the Latin letters of the immunoglobulin classes, e.g., μ to IgM. There are two types of light chains (denoted κ and λ), either of which may combine with any of the heavy chains and thus occur in any of the immunoglobulin classes. In human immunoglobulins, three of the classes (IgM, IgG, and IgA) have subclasses; i.e., there are several similar but distinct CH region genes in these classes. In addition, the λ light chain type has subtypes. The subclasses (subtypes) are denoted by numerical suffixes, e.g., IgG1 and γ1 subclasses and λ2 subtype. Immunoglobulins (monomeric IgM and IgD) first appear on the surface of B cells as antigen receptors. When a cell is activated by contact with antigen and differentiates into a plasma cell, the cell continues to produce the same L chain and VH region of the H chain, but gene rearrangement may occur to attach this VH region to a different CH region (class switching). Thus the secreted immunoglobulin may be of any class but has the same antigenic specificity as the antigen receptors of the parent B cell. In addition to the effects produced solely by the binding of antigen by antibody, e.g., viral neutralization or the inability of some bacteria to invade mucosal surfaces when coated by antibody, certain classes of antibodies can trigger other processes when bound to antigen: IgM and IgG activate the classic complement pathway, IgA and IgG activate the alternative pathway, and IgM, IgG1, and IgG3 act as opsonins, triggering phagocytosis of the bound antigens by macrophages and neutrophils. IgE has the unique function of mediating immediate hypersensitivity (q.v.) reactions; it binds to specific receptors on basophils and mast cells and triggers the release of mediators on contact with antigen. IgG is the only class transferred across the placenta, providing the fetus and neonate with protection against infection. See also immunoglobulin genes, under gene, homology region, under region, and allotype, idiotype, and isotype.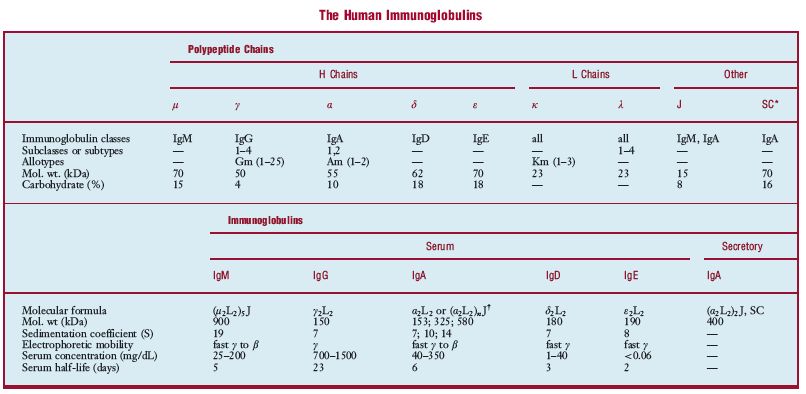
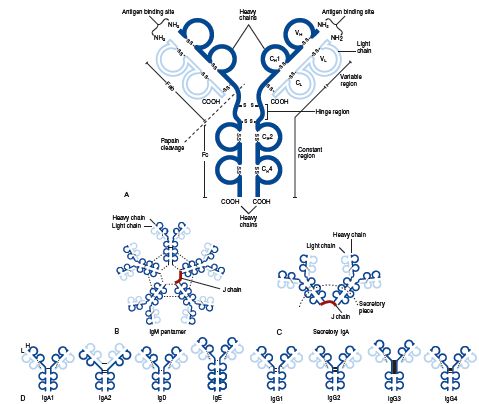
 Molecular structure of the five classes of immunoglobulins. (A), Schematic representation, using an IgG1 molecule, of the basic arrangement of heavy and light chains into the four-polypeptide chain unit, the antigen binding sites, the variable, constant, and hinge regions, and the site of papain cleavage generating the Fab and Fc fragments. Both intra- and interchain disulfide bonds are depicted (
Molecular structure of the five classes of immunoglobulins. (A), Schematic representation, using an IgG1 molecule, of the basic arrangement of heavy and light chains into the four-polypeptide chain unit, the antigen binding sites, the variable, constant, and hinge regions, and the site of papain cleavage generating the Fab and Fc fragments. Both intra- and interchain disulfide bonds are depicted ( SS
SS ). (B), IgM pentamer showing the arrangement of five four-chain monomers, interchain disulfide bonds (dotted lines), and the J chain. (C), Secretory IgA, here composed of IgA1 monomers, showing the position of the interchain disulfide bonds (dotted lines), J chain, and secretory piece. (D), Structure of IgA, IgD, IgE, and IgG subclasses, showing the interchain disulfide bonds (dotted lines).
). (B), IgM pentamer showing the arrangement of five four-chain monomers, interchain disulfide bonds (dotted lines), and the J chain. (C), Secretory IgA, here composed of IgA1 monomers, showing the position of the interchain disulfide bonds (dotted lines), J chain, and secretory piece. (D), Structure of IgA, IgD, IgE, and IgG subclasses, showing the interchain disulfide bonds (dotted lines).
Medical dictionary. 2011.